This can be accomplished using the standard modeling routine. The alignment should be as follows when the chimera is a combination of proteins A and B:
proteinA aaaaaaaaaaaaaaaaaaaaaaaaaaaa---------------------------------- proteinB ----------------------------bbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbb chimera aaaaaaaaaaaaaaaaaaaaaaaaaaaabbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbb
In the PIR format the alignment file is:
>P1;proteinA structureX:proteinA aaaaaaaaaaaaaaaaaaaaaaaaaaaa----------------------------------* >P1;proteinB structureX:proteinB ----------------------------bbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbb* >P1;chimera sequence:chimera aaaaaaaaaaaaaaaaaaaaaaaaaaaabbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbbb*
If no additional information is available about the relative orientation of the two domains the resulting model will probably have an incorrect relative orientation of the two domains when the overlap between A and B is non-existing or short. To obtain satisfactory relative orientation of modeled domains in such cases, orient the two template structures appropriately before the modeling.
The easiest way to achieve this is to not align that region of the template with the target sequence. If region 'bbbbbbbb' of the template should not be used as a template for region 'eeeee' of the target sequence the alignment should be like this:
template aaaaaaaaaaaaaaaaaaaaaaaa-----bbbbbbbbcccccccccccccccccccccccccccccc target ddddddddddddddddddddddddeeeee--------ffffffffffffffffffffffffffffff
The effect of this alignment is that no homology-derived restraints will be produced for region 'eeeee'.
MODELLER can restrain disulfides in two ways: automatically (model.patch_ss_templates() or model.patch_ss()) and manually (model.patch()).
If there is an equivalent disulfide bridge in any
of the templates aligned with the target, the model.patch_ss_templates()
command will generate appropriate disulfide bond restraints without
any other input from the user. This command is run automatically by the
'model' script used for comparative modeling. The restraints include
bond, angle and dihedral angle restraints. The SG -- SG atom pair
also becomes an excluded atom pair that is not checked for an atom-atom
overlap. The ![]() dihedral angle restraints will depend
on the conformation of the equivalent disulfides in the template
structure, as described in [Šali & Overington, 1994]. The command
model.patch_ss() is similar, except that the current structure of
MODEL, not templates, is used to guess the disulfide bonded
CYS - CYS pairs.
dihedral angle restraints will depend
on the conformation of the equivalent disulfides in the template
structure, as described in [Šali & Overington, 1994]. The command
model.patch_ss() is similar, except that the current structure of
MODEL, not templates, is used to guess the disulfide bonded
CYS - CYS pairs.
Explicit manual restraints can be added by the model.patch() command relying on the PRES DISU patching residue in the CHARMM topology file. When building comparative models with the automodel class, the `manual' disulfides should be defined in the automodel.special_patches() routine. The model.patch() command will establish the correct stereochemistry by relying on the CHARMM topology file and parameters to restrain the disulfide bond.
It is better to use model.patch_ss_templates() than model.patch() where possible because the dihedral angles are restrained more precisely by using the templates than the general rules of stereochemistry.
Some CHARMM parameter files have a multiple dihedral entry for
the disulfide dihedral angle ![]() that consists of three individual
entries with periodicities of 1, 2 and 3. This is why you see three
feature restraints for a single disulfide in the output of the
model.energy() command.
that consists of three individual
entries with periodicities of 1, 2 and 3. This is why you see three
feature restraints for a single disulfide in the output of the
model.energy() command.
from modeller.automodel import *
# Redefine the special_patches routine to include the additional disulfides
# (this routine is empty by default):
class mymodel(automodel):
def special_patches(self):
# A disulfide between residues 1 and 85 in chain A:
self.patch(residue_type='DISU', residue_ids=('1:A', '85:A'))
# A disulfide between residues 41 and 45 in chain B:
self.patch(residue_type='DISU', residue_ids=('41:B', '45:B'))
# This is as usual:
log.verbose()
env = environ()
a = mymodel(env, alnfile='align1.ali', knowns='templ1', sequence='targ1')
a.make()
MODELLER should usually be allowed to handle this automatically via the omega dihedral angle restraints, which are calculated by default.
from modeller.automodel import *
from modeller.scripts import cispeptide
# Redefine the special_restraints routine to force Pro to cis conformation:
# (this routine is empty by default):
class mymodel(automodel):
def special_restraints(self, aln):
cispeptide(self.restraints, atom_ids1=('O:4', 'C:4', 'N:5', 'CA:5'),
atom_ids2=('CA:4', 'C:4', 'N:5', 'CA:5'))
# This is as usual:
log.verbose()
env = environ()
a = mymodel(env, alnfile='align1.ali', knowns='templ1', sequence='targ1')
a.make()
Restraints can be read from a file by model.restraints.append(), calculated by model.restraints.make(), or added ``manually'' by model.restraints.add(). model.restraints.pick() picks those restraints for objective function calculation that restrain the selected atoms only, as specified in the selected atoms set 1. Initially, all atoms are selected; this can be changed by the model.pick_atoms() command. model.restraints.make() command for some restraint types (e.g., distance) constructs restraints of the selected type between the atoms in the selected atoms sets 2 and 3. The 'automodel.homcsr()' routine contains examples of the model.pick_atoms() command when generating restraints by model.restraints.make(). There are also commands for adding and unselecting single restraints, model.restraints.add() and model.restraints.unpick(), respectively. If you do model.restraints.condense(), the unselected restraints will be deleted. This is useful for getting rid of the unwanted restraints completely.
There are two different optimization approaches available within MODELLER: variable target function method (VTFM) with conjugate gradients (CG) [Šali & Blundell, 1993] and molecular dynamics (MD) with simulated annealing (SA) [Šali & Blundell, 1993]. They can both be done to a different degree (with more or less cycles of CG and MD, faster or slower schedule for VTFM and SA). The exact details are best obtained from the scripts themselves because a detailed description would probably be longer than the scripts. For example, the QUANTA and INSIGHTII implementations of MODELLER have these three levels of optimization: no optimization (only copying coordinates from templates and building the undefined atoms using internal geometry information from the RTF entries); only VTFM with CG; also MD with SA. Most of the time (70%) is spent on the MD&SA part. Our experience is that when MD&SA are used, if there are violations in the best of the 10 models, they probably come from an alignment error, not an optimizer failure (if there are no insertions longer than approximately 15 residues).
See chapter 2 for the variables that could be changed and for their possible values.
from modeller.automodel import * log.verbose() env = environ() a = automodel(env, alnfile='align1.ali', knowns='templ1', sequence='targ1') # Very thorough VTFM optimization: a.library_schedule = 1 a.max_var_iterations = 300 # Very thorough MD optimization: a.md_level = refine.very_slow # Repeat the whole cycle 3-times and do not stop unless obj.func. > 1E6 a.repeat_optimization = 3 a.max_molpdf = 1e6 a.make()
from modeller.automodel import * log.verbose() env = environ() env.io.hydrogen = env.io.hetatm = env.io.water = True a = allhmodel(env, alnfile='align1.ali', knowns='templ1', sequence='targ1') a.make()
Water molecules are indicated by 'w' in the alignment file and the special block residue ('BLK') that does not have entries in the residue topology and parameter libraries is indicated by '.'
See Section 3.5.1 for information about block residues.
from modeller.automodel import * log.verbose() env = environ() env.io.hetatm = env.io.water = True a = automodel(env, alnfile='align1.ali', knowns='templ1', sequence='targ1') a.make()
The alignment file:
>P1;templ1 structureX:templ1:1::10:: FAYVI/.wwww* >P1;targ1 sequence:targ1:1::8:: -GWIV/.ww-w*
This is a painful area in all molecular modeling programs. However, CHARMM and X-PLOR provide a reasonably straightforward solution via the residue topology and parameter libraries. MODELLER uses CHARMM topology and parameter library format and also extends the options by allowing for a generic ``BLK'' residue type (Section 3.5.1). This BLK residue type circumvents the need for editing any library files, but it is not always possible to use it. Due to its conformational rigidity, it is also not as accurate as a normal residue type. In order to define a new residue type in the MODELLER libraries, you have to follow the series of steps described below. As an example, we will define the ALA residue without any hydrogen atoms. You can add an entry to the MODELLER topology or parameter file; you can also use your own topology or parameter files. For more information, please see the CHARMM manual.
- Define the new residue entry in the residue topology file (RTF),
say 'top_heav.lib'.
RESI ALA 0.00000 ATOM N NH1 -0.29792 ATOM CA CT1 0.09563 ATOM CB CT3 -0.17115 ATOM C C 0.69672 ATOM O O -0.32328 BOND CB CA N CA O C C CA C +N IMPR C CA +N O CA N C CB IC -C N CA C 1.3551 126.4900 180.0000 114.4400 1.5390 IC N CA C +N 1.4592 114.4400 180.0000 116.8400 1.3558 IC +N CA *C O 1.3558 116.8400 180.0000 122.5200 1.2297 IC CA C +N +CA 1.5390 116.8400 180.0000 126.7700 1.4613 IC N C *CA CB 1.4592 114.4400 123.2300 111.0900 1.5461 IC N CA C O 1.4300 107.0000 0.0000 122.5200 1.2297 PATC FIRS NTER LAST CTER
You can obtain an initial approximation to this entry by defining the new residue type using the residue type editor in QUANTA and then writing it to a file.
The RESI record specifies the CHARMM residue name, which can be up to four characters long and is usually the same as the PDB residue name (exceptions are the potentially charged residues where the different charge states correspond to different CHARMM residue types). The number gives the total residue charge.
The ATOM records specify the IUPAC (i.e., PDB) atom names and the CHARMM atom types for all the atoms in the residue. The number at the end of each ATOM record gives the partial atomic charge.
The BOND records specify all the covalent bonds between the atoms in the residue (e.g., there are bonds CB-CA, N-CA, O-C, etc.). In addition, symbol '+' is used to indicate the bonds to the subsequent residue in the chain (e.g., C - +N). The covalent angles and dihedral angles are calculated automatically from the list of chemical bonds.
The IMPR records specify the improper dihedral angles, generally used to restrain the planarity of various groups (e.g., peptide bonds and sidechain rings). See also below.
The IC (internal coordinate) records are used for constructing the initial Cartesian coordinates of a residue. An entry
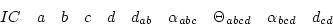
specifies distances
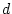 , angles
, angles 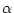 , and either dihedral angles
or improper dihedral angles
, and either dihedral angles
or improper dihedral angles  between atoms
between atoms 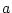 ,
, 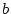 ,
, 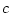 and
and
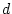 , given by their IUPAC names. The improper dihedral angle
is specified when the third atom,
, given by their IUPAC names. The improper dihedral angle
is specified when the third atom, 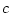 , is preceded by a star,
'*'. As before, the '-' and '+' pre-fixes for the atom names select
the corresponding atom from the preceding and subsequent residues,
respectively. The distances are in angstroms, angles in degrees.
The distinction between the dihedral angles and
improper dihedral angles is unfortunate since they are the
same mathematically, except that by convention when using the
equations, the order of the atoms for a dihedral angle is
, is preceded by a star,
'*'. As before, the '-' and '+' pre-fixes for the atom names select
the corresponding atom from the preceding and subsequent residues,
respectively. The distances are in angstroms, angles in degrees.
The distinction between the dihedral angles and
improper dihedral angles is unfortunate since they are the
same mathematically, except that by convention when using the
equations, the order of the atoms for a dihedral angle is 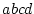 and for an improper dihedral angle it is
and for an improper dihedral angle it is 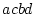 .
.
The PATC record specifies the default patching residue type when the current residue type is the first or the last residue in a chain.
- You have to make sure that all the CHARMM atom types of the
new residue type occur in the MASS records at the beginning
of the topology library: Add your entry at the end of the MASS list if
necessary. If you added any new CHARMM atom types, you also have to
add them to the radii libraries, 'modlib/radii.lib' and
'modlib/radii14.lib'. These libraries list the atomic radii for the
different topology models, for the long range and 1-4 non-bonded
soft-sphere terms, respectively. The full names of the files that are
used during calculation are given by the environment variables
$RADII_LIB and $RADII14_LIB.
- Optionally, you can add the residue entry to the library of
MODELLER topology models, 'modlib/models.lib'. The runtime
version of this library is specified by the environment variable
$MODELS_LIB. This library specifies which subsets of atoms
in the residue are used for each of the possible topologies.
Currently, there are 10 topologies selected by topology.submodel
(3 is default):
1 ALLH all atoms 2 POL polar hydrogens only 3 HEAV non-hydrogen atoms only 4 MCCB non-hydrogen mainchain (N, C, CA, O) and CB atoms 5 MNCH non-hydrogen mainchain atoms only 6 MCWO non-hydrogen mainchain atoms without carbonyl O 7 CA CA atoms only 8 MNSS non-hydrogen mainchain atoms and disulfide bonds 9 CA3H reduced model with a small number of sidechain interaction centers 10 CACB CA and CB atoms only The Ala entry is:
# ALLH POLH HEAV MCCB MNCH MCWO CA MNSS CA3H CACB * RESI ALA ATOM NH1 NH1 NH1 NH1 NH1 NH1 #### NH1 #### #### ATOM H HN #### #### #### #### #### #### #### #### ATOM CT1 CT1 CT1 CT1 CT1 CT1 CT1 CT1 CAH CT1 ATOM HB #### #### #### #### #### #### #### CH3E #### ATOM CT3 CT3 CT3 CT3 #### #### #### #### #### CT2 ATOM HA #### #### #### #### #### #### #### #### #### ATOM HA #### #### #### #### #### #### #### #### #### ATOM HA #### #### #### #### #### #### #### #### #### ATOM C C C C C C #### C #### #### ATOM O O O O O #### #### O #### ####The residue entries in this library are separated by stars. The '####' string indicates a missing atom. The atom names for the present atoms are arbitrary. The order of the atoms must be the same as in the CHARMM residue topology library. If a residue type does not have an entry in this library, all atoms are used for all topologies.
- You have to add the new residue type to the residue type library,
'modlib/restyp.lib'. The execution version of this file is
specified by the environment variable $RESTYP_LIB. For the
ALA residue,
1 | ALA | A | ALA | alanine
You would generally add the new residue type at the end of the file. There are 5 fields in each line, separated by the '|' characters. The first field is an integer index corresponding to the integer residue type. The standard residue types have their indices smaller than 24. These are also the indices corresponding to the residue-residue substitution matrices. The second field contains the list of equivalent PDB or IUPAC 3-character residue names, used in the PDB files. A list rather than a single name is allowed because PDB can unfortunately use different names for the same residue type (e.g., water can be HOH, WAT, etc.). The third field gives a single character code for the residue type, which is used in the alignment file. This does not have to be unique, but if it is not unique you cannot use it in the alignment file. Any ASCII character is fine, it does not have to be a letter. If you run out of characters you can re-define the existing ones that you do not need. The fourth field gives the four-character CHARMM residue name, as specified in the RESI record of the topology library. The last field contains an optional comment.
Every residue in the CHARMM topology file has to have an entry in the $RESTYP_LIB library, but not every residue entry in the $RESTYP_LIB library needs an entry in the residue topology file.
When you are adding a new residue type, you have to hope that the maximal number of residue types is not over-reached. If it is, a fatal error is reported at the beginning of the execution. To solve this problem, you could delete some of the un-needed existing residue types in the $RESTYP_LIB file, rather than re-compile the program with larger array sizes. You can also read your own residue type library by the libraries.read_restyp() command.
- In general, when you add a new residue type, you also add new
chemical bonds, angles, dihedral angles, improper dihedral angles,
and non-bonded interactions,
new in the sense that a unique combination of CHARMM atoms types
is involved whose interaction parameters are not yet specified in the
parameter library (see also Section 3.5.1).
In such a case, you will get a number of
warning and/or error messages when you generate the stereochemical
restraints by the model.restraints.make() command. These messages
can sometimes be ignored because MODELLER will guess the
values for the missing parameters from the
current Cartesian coordinates of the model. When this is not accurate
enough or if the necessary coordinates are undefined
you have to specify the parameters explicitly in the parameter
library. Search for BOND, ANGL, DIHE, and IMPR sections in the
parameters library file and use the existing entries to guess your
new entries. Note that you can use dummy atom types 'X' to create
general dihedral (i.e., X A A X) and improper dihedral angle (i.e.,
A X X A) entries, where A stands for any of the real CHARMM
atom types.
For the dihedral angle cosine terms, the CHARMM convention for the phase
is different for 180
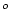 from MODELLER's (Eq. 5.57).
If you use non-bonded Lennard-Jones terms, you also have
to add a NONB entry for each new atom type. If you use the default soft-sphere
non-bonded restraints, you have already taken care of it by
adding the new atom types to the $RADII_LIB and $RADII_LIB
libraries.
from MODELLER's (Eq. 5.57).
If you use non-bonded Lennard-Jones terms, you also have
to add a NONB entry for each new atom type. If you use the default soft-sphere
non-bonded restraints, you have already taken care of it by
adding the new atom types to the $RADII_LIB and $RADII_LIB
libraries.
This is even messier than defining a new residue type. As an example, we will define the patching residue for establishing a disulfide bond between two CYS residues.
PRES DISU -0.36 ! Patch for disulfides. Patch must be 1-CYS and 2-CYS. ATOM 1:CB CT2 -0.10 ! ATOM 1:SG SM -0.08 ! 2:SG--2:CB-- ATOM 2:SG SM -0.08 ! / ATOM 2:CB CT2 -0.10 ! -1:CB--1:SG DELETE ATOM 1:HG DELETE ATOM 2:HG BOND 1:SG 2:SG IC 1:CA 1:CB 1:SG 2:SG 0.0000 0.0000 180.0000 0.0000 0.0000 IC 1:CB 1:SG 2:SG 2:CB 0.0000 0.0000 90.0000 0.0000 0.0000 IC 1:SG 2:SG 2:CB 2:CA 0.0000 0.0000 180.0000 0.0000 0.0000
The PRES record specifies the CHARMM patching residue type (up to four characters). As for the normal RESI residue types, patching residue types also have to be defined in the residue type library, 'modlib/restyp.lib'.
The ATOM records have the same meaning as for the RESI residue types described above. The extension is that the IUPAC atom names (listed first) must be pre-fixed by the index of the residue that is patched. In this example, there are two CYS residues that are patched, thus the prefixes 1 and 2. When using the model.patch() command, the order of the patched residues specified by residue_ids must correspond to these indices (this is only important when the patch is not symmetric, unlike the 'DISU' patch in this example).
DELETE records specify the atoms to be deleted, the two hydrogens bonded to the two sulfurs in this case.
The BOND and IC (internal coordinate) records are the same as those for the RESI residues, except that the atom names are prefixed with the patched residue indices.
Yes. There are 'ALPHA', 'STRAND' and 'SHEET' restraint types that the model.restraints.make() command can generate. One specifies the segment which is then restrained to the specified secondary structure conformation. For example,
from modeller.automodel import *
# Redefine the special_restraints routine to include the secondary
# structure restraints (this routine is empty by default):
def mymodel(automodel):
def special_restraints(self, aln):
rsr = self.restraints
# An alpha-helix:
rsr.make(aln, restraint_type='ALPHA', residue_ids=('20', '30'))
# Two strands:
rsr.make(aln, restraint_type='STRAND', residue_ids=('1', '6'))
rsr.make(aln, restraint_type='STRAND', residue_ids=('9', '14'))
# An anti-parallel sheet:
rsr.make(aln, restraint_type='SHEET', atom_ids=('N:1', 'O:14'),
sheet_h_bonds=-5)
# This is as usual:
log.verbose()
env = environ()
a = automodel(env, alnfile='align1.ali', knowns='templ1', sequence='targ1')
a.make()
This is probably because the N-terminus is patched by default with
the NTER patching residue (corresponding to -NH3![]() ) and a patched
residue must not be patched again. The solution is to turn the default
patching off by env.patch_default = False before the
model.generate_topology() command is called.
) and a patched
residue must not be patched again. The solution is to turn the default
patching off by env.patch_default = False before the
model.generate_topology() command is called.
Yes. You do not have to do anything special.
First, check for the error messages by searching for string '_E>''. These messages can only rarely be ignored. Next, check for the warning messages by searching for string '_W>''. These messages can almost always be ignored. If everything is OK so far, the most important part of the log file is the output of the model.energy() command for each model. This is where the violations of restraints are listed. When there are too many too violated restraints, more optimization or a different alignment is needed. What is too many and too much? It depends on the restraint type and is best learned by doing model.energy() on an X-ray structure or a good model to get a feel for it. You may also want to look at the output of command alignment.check(), which should be self-explanatory. I usually ignore the other parts of the log file.
The best way to prevent knots is to start with a starting structure that is as close to the desired final model as possible. Other than that, the only solution at this point is to calculate independently many models and hope that in some runs there won't be knots. Knots usually occur when one or more neighboring long insertions (i.e., longer than 15 residues) are modeled from scratch. The reason is that an insertion is build from a randomized distorted structure that is located approximately between the two anchoring regions. Under such conditions, it is easy for the optimizer to ``fall'' into a knot and then not be able to recover from it. Sometimes knots result from an incorrect alignment, especially when more than one template is used. When the alignment is correct, knots are a result of optimization not being good enough. However, making optimization more thorough by increasing the CPU time would not be worth it on the average as knots occur relatively infrequently. The excluded volume restraints are already included in the standard comparative modeling routine.
Usually more than that (dozens if you want just to detect reliable similarity, and even more if you want a real model). It is good to have at least 35-40% sequence identity to build a model. Sometimes even 30% is OK.
No; Modeller is run from the command line, and uses a Python script to direct it. However, a graphical interface to Modeller is commercially available from Accelrys, as part of Discovery Studio Modeling 1.1, at http://www.accelrys.com/dstudio/ds_modeling/ds_modeler.html.
When you give MODELLER an alignment, it also needs to read the structure of the known proteins (templates) from PDB files. In order to correctly match coordinates to the residues specified in the alignment, the sequences in the PDB file and the alignment file must be the same (although obviously you can add gap or chain break characters to your alignment). If they are not, you see this error. (Note that MODELLER takes the PDB sequence from the ATOM and HETATM PDB records, not the SEQRES records.)
To see the sequence that MODELLER reads from the PDB file '1BY8.pdb', use this short script to produce a '1BY8.seq' sequence file:
env = environ() code = '1BY8' mdl = model(env, file=code) aln = alignment(env) aln.append_model(mdl, align_codes=code) aln.write(file=code+'.seq')